Yeap, that’s right. Fluoride is good for your teeth. If you’ve ever been to the dentist, or know of anyone working in the dental industry, they would have probably ‘drilled’ this into you, saying that fluoride can help to strengthen your teeth, and prevents holes (dental decay) from developing in your teeth…
In this post, we’ll discuss about why and how fluoride actually works, so that it is not just an instruction to follow blindly, but to make sense of it all.
In order to understand it, we must first look at the tooth itself. The outermost layer of the tooth is called the ENAMEL. Enamel is made up of tiny crystals called HYDROXY-APATITE. Hydroxyapatite is a very strong structure and gives enamel most of its strength. However, this crystal structure can break down when it gets exposed to an acidic environment.
Just a bit of theory: When discussing about acidity, we normally use a term called ‘pH’. pH is usually given a number between 1 – 14. A pH of 7 means it is neutral (neither acidic or alkaline). Any number smaller than pH 7 is considered acidic, and above the pH 7 is alkaline.
The pH in the mouth is usually in a neutral state of 7. The mouth/teeth becomes an acidic environment when we eat sweet and sour foods and drinks. The problem with this is that the hydroxyapatite crystals on the enamel start to dissolve at pH 5.5 and below! This process is called DEMINERALISATION.
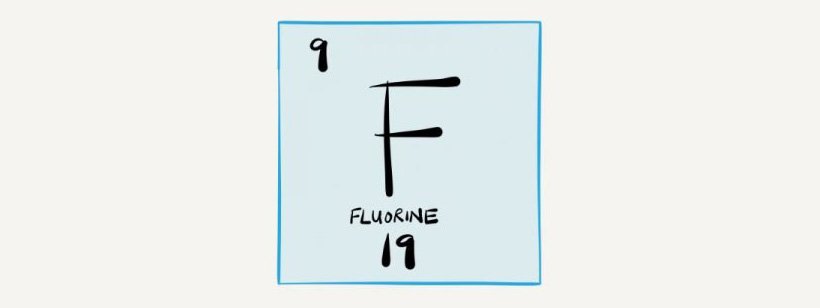
So here is where fluoride comes in to save the day. When teeth are exposed to fluoride, that hydroxyapatite layer changes to become FLUOR-APATITE (!). Almost like Clark Kent taking of his glasses and changing into Superman…
 Fluorapatite only starts to dissolve at a pH of 4.5, which makes the tooth surface now more resistant to demineralisation and dissolution, and therefore much stronger than before.
Fluorapatite only starts to dissolve at a pH of 4.5, which makes the tooth surface now more resistant to demineralisation and dissolution, and therefore much stronger than before.
 The graph shows the change in acidity over time after a 1 minute sugary mouth rinse. Firstly, it takes about 30 – 60 mins to recover back to ph 7. Secondly, fluorapatite (pH 4.5) dissolves much less than hydroxyapatite (pH 5.5).
Knowing this, it makes sense to keep our teeth exposed to fluoride as much as possible to maintain that strong layer of fluorapatite. So, here is some advice about fluoride use:
The graph shows the change in acidity over time after a 1 minute sugary mouth rinse. Firstly, it takes about 30 – 60 mins to recover back to ph 7. Secondly, fluorapatite (pH 4.5) dissolves much less than hydroxyapatite (pH 5.5).
Knowing this, it makes sense to keep our teeth exposed to fluoride as much as possible to maintain that strong layer of fluorapatite. So, here is some advice about fluoride use:
 Fluorapatite only starts to dissolve at a pH of 4.5, which makes the tooth surface now more resistant to demineralisation and dissolution, and therefore much stronger than before.
Fluorapatite only starts to dissolve at a pH of 4.5, which makes the tooth surface now more resistant to demineralisation and dissolution, and therefore much stronger than before.
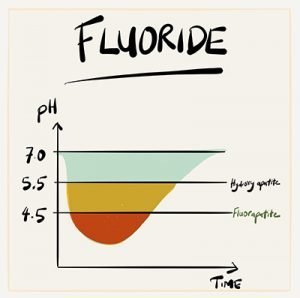 The graph shows the change in acidity over time after a 1 minute sugary mouth rinse. Firstly, it takes about 30 – 60 mins to recover back to ph 7. Secondly, fluorapatite (pH 4.5) dissolves much less than hydroxyapatite (pH 5.5).
Knowing this, it makes sense to keep our teeth exposed to fluoride as much as possible to maintain that strong layer of fluorapatite. So, here is some advice about fluoride use:
The graph shows the change in acidity over time after a 1 minute sugary mouth rinse. Firstly, it takes about 30 – 60 mins to recover back to ph 7. Secondly, fluorapatite (pH 4.5) dissolves much less than hydroxyapatite (pH 5.5).
Knowing this, it makes sense to keep our teeth exposed to fluoride as much as possible to maintain that strong layer of fluorapatite. So, here is some advice about fluoride use:
- Use fluoride dental products regularly – toothpaste, mouthwash, gels, tooth mousse
- After brushing your teeth, spit out the excess toothpaste, but don’t rinse. Leave a small layer of toothpaste in the mouth. Sounds yucky, but keeping some toothpaste (which contains fluoride) in contact with the teeth helps! Fluoride only works when it is exposed to the teeth, so it doesn’t really work much if you swallow it, or rinse it all out.
- For kids under 6 years of age, use a pea-sized amount of toothpaste. Try to avoid your kids from swallowing toothpaste, because excessive fluoride can interfere with the development of the adult teeth, causing mottling (fluorosis). In adults, teeth are already developed, so there is no problems with fluorosis.
- Visit your dentist every 6 months for an application of fluoride varnish
- Drink water. In Australia, most of the tap water is fluoridated. Avoid fizzy drinks and fruit juices as much as possible!