- amount of sugar, and the
- time/frequency of sugars in the mouth,
 As you know, the mouth is the beginning of the alimentary tract, and has a whole host of different types of bacteria. The combination of the different bacteria and saliva is called a biofilm, which coats the many surfaces of the mouth, including the gums, tongue and TEETH.
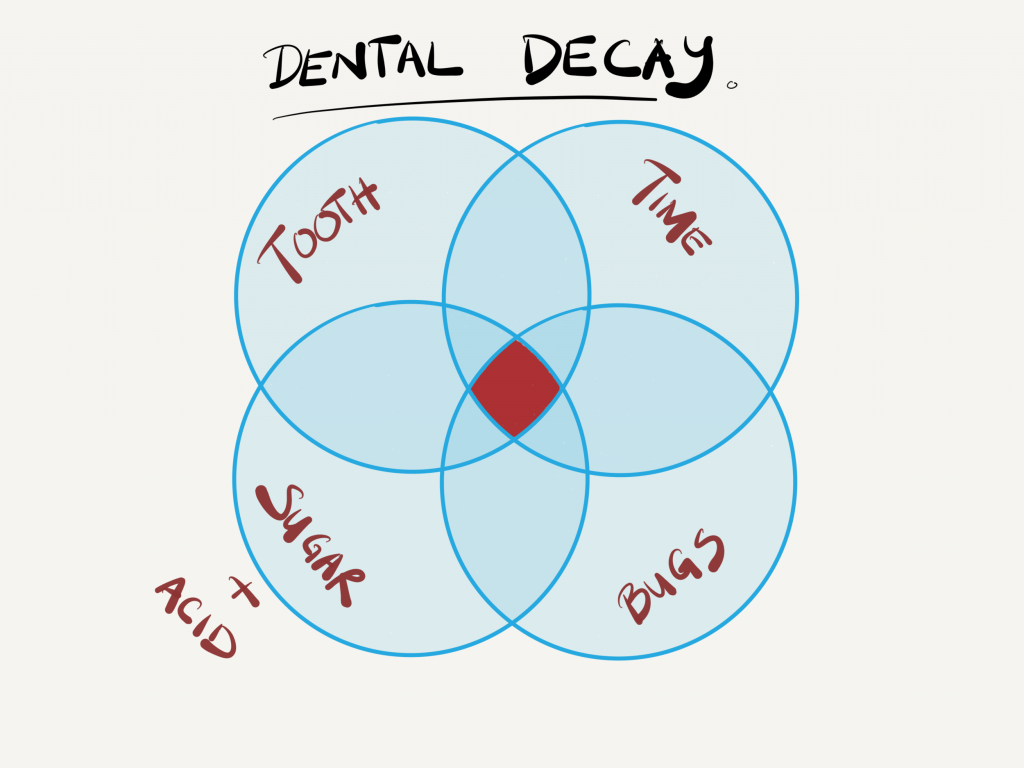
So what does the bacteria have anything to do with holes (decay) in teeth?
Well, there are some bacteria in the mouth that love sugar as much as we do. However, it is their by-products that we need to be aware of: ACID.
In fact, these bacteria, called ‘cariogenic’ bacteria, can secrete the acids within 1 -3 minutes after being in contact with sugar! And, as you know, acids are erosive on certain materials, the tooth surface (enamel and dentine) being one of them!
Yes, the enamel, being the hardest and most mineralised substance (made of calcium and phosphate ions) in our body can dissolve in an acidic environment quite readily.
At it’s molecular level, the enamel surface is made of calcium and phosphate ions, arranged in a crystalline formation called hydroxyapatite. These calcium and phosphate ions can start to dissolve and break down when the acidic environment goes below pH 5.5. This process is called ‘DE-mineralisation’.
Acidity increases as the pH** value goes down (anything below the pH of 7 is considered acidic), and the pH value of the acids produced by the bacteria in the mouth can go as low as pH 4.5! If this acidic environment is constantly present, holes (dental decay) will start…Be aware that, this acidic environment can take about 30-60 minutes to go back up to pH 7 again. So be careful!
As you know, the mouth is the beginning of the alimentary tract, and has a whole host of different types of bacteria. The combination of the different bacteria and saliva is called a biofilm, which coats the many surfaces of the mouth, including the gums, tongue and TEETH.
So what does the bacteria have anything to do with holes (decay) in teeth?
Well, there are some bacteria in the mouth that love sugar as much as we do. However, it is their by-products that we need to be aware of: ACID.
In fact, these bacteria, called ‘cariogenic’ bacteria, can secrete the acids within 1 -3 minutes after being in contact with sugar! And, as you know, acids are erosive on certain materials, the tooth surface (enamel and dentine) being one of them!
Yes, the enamel, being the hardest and most mineralised substance (made of calcium and phosphate ions) in our body can dissolve in an acidic environment quite readily.
At it’s molecular level, the enamel surface is made of calcium and phosphate ions, arranged in a crystalline formation called hydroxyapatite. These calcium and phosphate ions can start to dissolve and break down when the acidic environment goes below pH 5.5. This process is called ‘DE-mineralisation’.
Acidity increases as the pH** value goes down (anything below the pH of 7 is considered acidic), and the pH value of the acids produced by the bacteria in the mouth can go as low as pH 4.5! If this acidic environment is constantly present, holes (dental decay) will start…Be aware that, this acidic environment can take about 30-60 minutes to go back up to pH 7 again. So be careful! 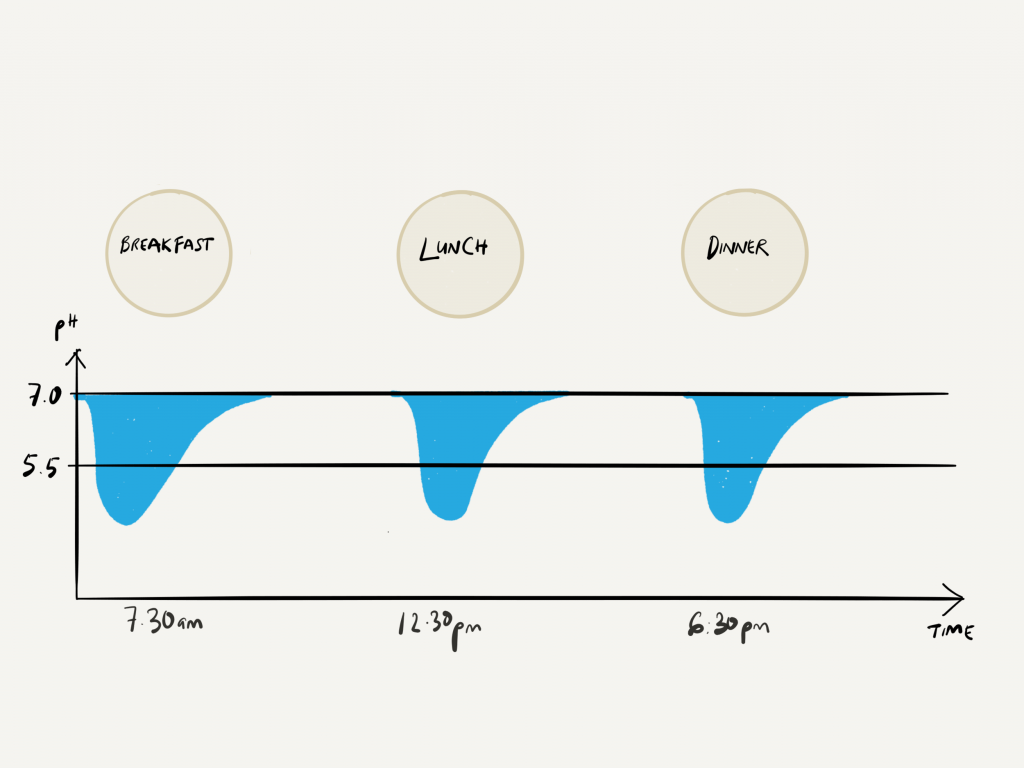
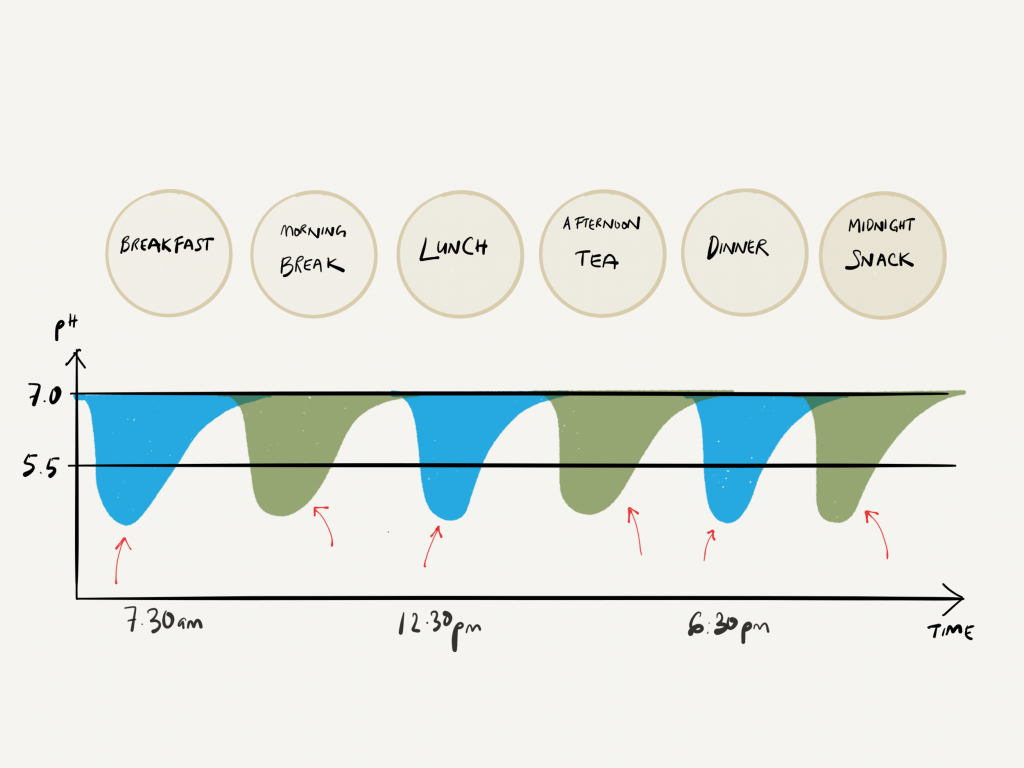
So with this information about the acids produced by the bacteria and its effect on the teeth, below are some tips to help reduce/prevent the potential breakdown (demineralisation) of the surfaces of the teeth. Don’t worry, there is still hope! Some of these tips may be familiar from previous blog posts:
- Reduce the sugar intake, and more importantly, the time that the sugar is in the mouth. The less amount and time sugars are in the mouth, the less the bacteria can utilise to form the acids. This is the main reason for avoiding sugary snacks in between meals. If you do have something sweet, have it closer to your main meal time (e.g. a sweet dessert straight after dinner).
- In terms of foods, be aware that carbohydrates (e.g. bread, starches, pasta, etc..) can be broken down into sugars by the enzymes in the saliva in the mouth. These sugars can be utilised by the bacteria as well. So it’s not just the sweet stuff…
- Speaking of acidic environments, fizzy drinks and fruit juices can be very acidic. Combined with the high level of sugars in these drinks, your teeth don’t stand a chance! Did you know that a lot these fizzy drinks can have a pH as low as pH 2.5?! Just google ‘pH of fizzy drinks’ and you’ll be amazed how low they can go…Always use a straw, and swallow it straight away. Try to avoid slushing it round the mouth!
- More saliva, the better. Saliva is naturally present in the mouth for many reasons. However, relating to the discussion about acids, saliva is slightly alkaline, and acts as a buffer to acids! Saliva also has a rich source of calcium and phosphate that can replenish those lost ions by a process called ‘RE-mineralisation’. This is why chewing sugar-free chewing gum is advised after foods. The act of chewing can help increase the flow of saliva, reducing the acidic environment in the mouth.
- Cleaning your teeth, by brushing and flossing them thoroughly, will reduce the amount of food stuck on and between your teeth – reducing the chances of bacteria utilising it. Just imagine the amount of acid produced by the bacteria feasting on some left over biscuit crumbs between your teeth the whole night!
- Fluoride can add a layer of extra protection on the tooth surface (called a fluoroapatite layer) to protect from demineralisation. This has been discussed in more detail in a previous post. So make sure you use that fluoride toothpaste and fluoride mouth rinse regularly – especially at night.
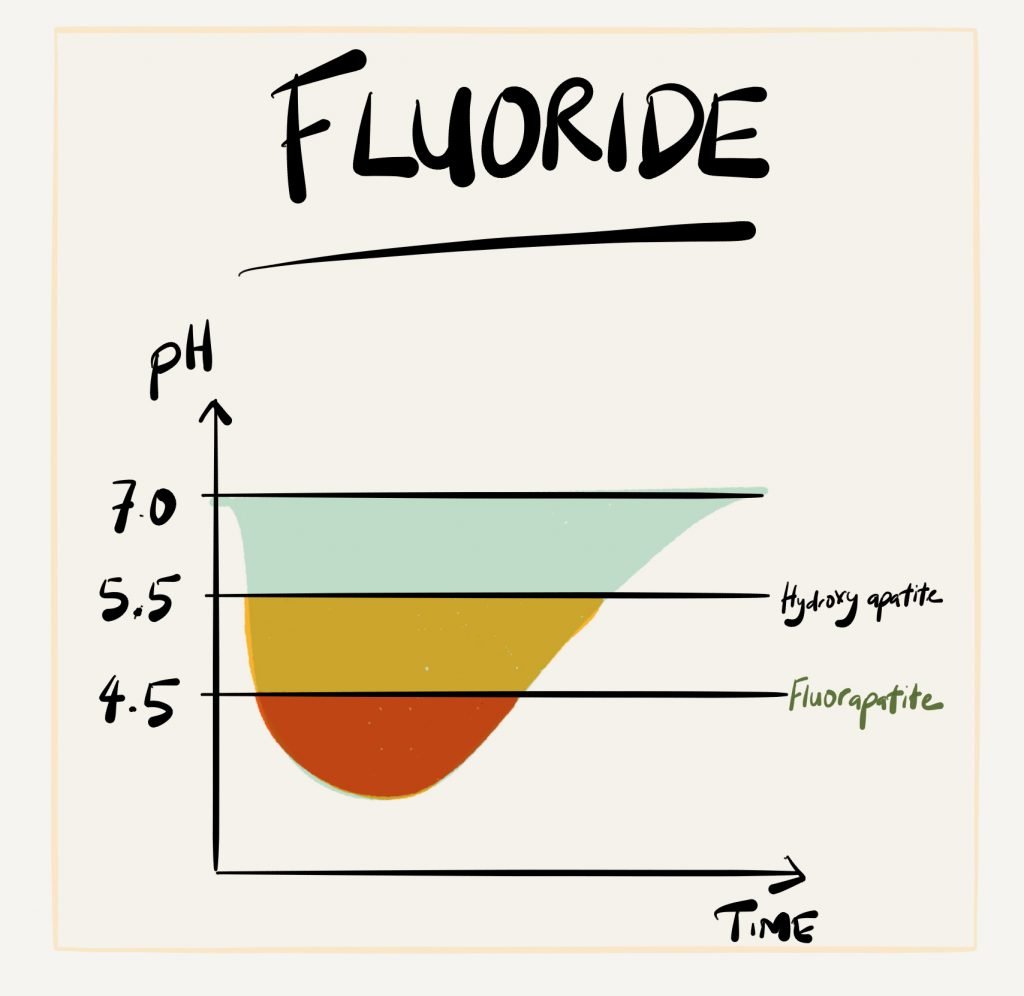
No, sterilising, or removing all the bacteria the mouth is not an option at this point (I know you were thinking it!). In the end, it will depend on YOU: the foods you eat, and the effectiveness of your cleaning will effect the health of your teeth and gums. So do it well!
* The most common cariogenic bacteria in the mouth is the streptococcus mutants, which metabolises sucrose and produces lactic acid as an end product.
** pH is the measure of how acidic or alkaline a solution is. It is measured as a number between 1 – 14, where the pH value of 7 is considered neutral (neither acidic or alkaline). A pH value below 7 is acidic, and above pH 7 is alkaline.